—
Note from the Pasteur Brewing Editor:
The “forcing process” was designed to estimate the relative keeping quality of beers, by investigating the production of healthy yeast and/or bacteria in beer samples, prior to bottling. This is a highly technical process and requires efficient laboratory equipment.
—
Originally published in “The Microscope in the Brewery and Malt-House” in 1889.
CHAPTER VIII
The Forcing Process
We have already in Chapters III and IV made frequent reference to Pasteur’s classical researches into the fermentation of Beer and Wine, and we now wish to explain how the methods first employed in those researches, may with advantage be practically applied by the scientific brewer to the regular examination of his product.
Briefly, Pasteur’s method of investigation may be said to consist of experimental fermentations with fermentable liquids which had been completely sterilized by repeated boiling in glass vessels, whose outlets were either shut off from the air, or so plugged with cotton wool as only to admit thoroughly filtered air. When the liquid in the glass vessels was found to remain free from change, it was inoculated with minute portions of the purest growths obtainable by the methods employed. We thus purposely define Pasteur’s pure growths, because Hansen’s recent work has shown conclusively that the separation of the Saccharomyces by shape alone is impossible, and it is therefore more than probable that many of Pasteur’s experiments were conducted with more than one variety of yeast.
One of the chief results of Pasteur’s work was, that working under the conditions above-named, his fermented liquids usually remained free from Bacteria, and therefore free from those acid changes which under less favourable conditions are found to accompany, or more correctly speaking follow, alcoholic fermentation. Pasteur had very carefully investigated some of these Bacteria, more especially those producing Acetic and Lactic acids, and it was the consideration of his published works on Vinegar and Wine that induced the leading Burton chemists to apply his methods of investigation to the systematic examination of Beer, prior to the publication of the well known work “Etudes sur la Biere,” in 1876. One of the most earnest and indefatigable workers in this direction was Horace T. Brown, and he may be said to have first systematized the method of beer examination by “forcing.”
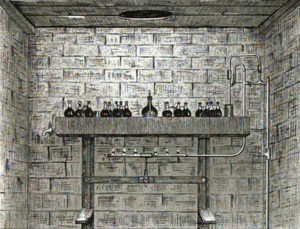
It is to the consideration of this method of testing the keeping qualities of ales and worts—depending as it does so materially on the use of the microscope—that we intend to devote this chapter; and in the first place we will describe the piece of apparatus employed first in Burton, and now generally used throughout England, known as the Forcing Tray [Plate XI.] It is an oblong vessel, made preferably of copper ; the size varies somewhat, but we find the following dimensions very convenient:—2 ft. 9 in. by i ft. 9 in., and 3 in. deep; the upper surface may be turned up about ^ in. all round to form a rim.
The want of attention to certain details, and to the fitting of various accessories, may very considerably vitiate any results obtained by the use of this apparatus; so that we shall now describe in some detail the apparatus itself and the method of using it, before entering upon a description of the varied microscopical observations, which are the principal sources of information gained by its use.
It is advisable to have an oblong sheet of thin copper or block tin, brazed or soldered on the under side of the tray, in order that the gases given off by the burner used for heating it, may not corrode the actual surface of the tray. When this protecting piece is found to be seriously corroded it may be easily replaced by a fresh one. Inside the tray it is usual to have another sheet of copper covering the central part, and supported about \ inches from the bottom; this is called the Disperser, and extends to within about 3 or 4 inches of the sides of the tray; its purpose is to prevent the water directly heated by the burners rising at once to the top of the tray, and so causing an unequal heating of its upper surface. A fair sized tubular opening should be provided for filling the tray, and this may be loosely covered by a cap, or plugged with cotton wool. A tap or bib-cock may conveniently be fixed at one end or underneath for emptying when repairs are required.
The heating, which is all important, should whenever possible be with gas, as the regulation of any other source of heat is difficult. A piece of ordinary f-inch gas-piping about one foot long, with six porcelain-tipped nipples (size No. 2), screwed in at equal distances, is as suitable a burner as any (see illustration), and should the tray be square the gas piping may with advantage be bent into a circle. This burner is fixed so that the surfaces of the nipples are not less than three inches from the plate on the underside of the tray, and it is directly connected with the Regulator by India-rubber or “composition” tubing.
The usual form of Regulator is that known as Page’s, which may be described as follows:
The bulb B and about an inch of the tube A is filled with clean mercury, which may conveniently be done by pouring small quantities of mercury at a time, into a small cone of stout writing paper with a good sized pinhole in the point, placed in the upper end of the tube A, or in the side tube K; closing the opening not used, with the finger. The regulator is now placed in a small flask of water on the tray, as shown in Plate XI.
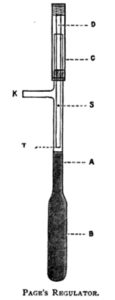
The tray being quite filled with water at a temperature sufficient to keep the thermometer on the tray about two degrees below the required temperature, a pint or two of water may be drawn off to allow for expansion. The sliding tube C of the regulator is now connected by India-rubber tubing to the gas main, and the outlet tube K similarly connected to the burner; gas passes to the latter down the quill tube D, a small amount, sufficient only to keep the burner from going out, going direct through the pin hole S, the main portion through the end T— which in some cases is beveled—and up the tube A into K. Under these conditions too much gas passes to the burners, and the temperature of the tray rises. When the thermometer on the tray indicates the required temperature (about 8˚ F.), the sliding tube C is pressed down until the lower end of the quill tube T just touches the surface of the mercury. If too much gas is thus cut off, the temperature of the tray falls slightly, the mercury in the regulator contracts and falls in the tube A, thus allowing more gas to pass through the quill tube at T, and so to the burners.
In an older form of this regulator, which is somewhat more reliable with a large amount of variation in the gas pressure (frequently the case in large works like breweries), the small hole in the side of the quill tube is replaced by a metal H piece with a tap in the centre; the two lower ends are connected respectively with the main and the burner, and the two upper ends with the quill tube and the side tube of the regulator. In use, the tap in the H piece is opened sufficiently to allow the same amount of gas to pass direct to the burner, as does the small hole in the previously described instrument; the remainder has first to pass through the quill tube of the regulator, the only passage being through the end T, which is directly controlled by the expansion and contraction of the mercury in the bulb. A modification of this arrangement is shown in Plate XL, the main gas pipe forming practically one limb of the H piece.
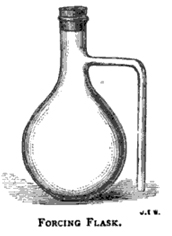
The flasks hold preferably about 120 cubic centimetres, and we find the pattern given in Fig. 26 more satisfactory to work with than any other; the old form with loose side tubes to be joined by India-rubber tubing being the cause of much waste of time, and therefore not to be recommended. In cleaning these flasks the greatest care must be taken to put no pressure on the side tube, as it is very liable to break off: well made flasks, however, stand ordinary handling well, and we have many that have been in regular use several years.
It will be as well now to consider the precautions necessary when collecting samples:
Beer samples are best taken from the racking vessel: when beer is racked direct from cleansing casks, the samples may be taken from them or from the trade casks as soon as filled.
Ordinary 10 oz. stoppered bottles are quite suitable for collecting these samples, and may also be used for obtaining the sediment of the racking sample to be microscopically examined.
Each bottle should be carefully cleaned, and first rinsed out with the beer to be sampled, before filling; it should also be labeled as soon as taken.
With regard to cleaning: The bottles should be thoroughly rinsed round with a solution of caustic soda, then well washed out and allowed to drain neck downwards; forcing-flasks may have a few cubic centimetres of a dilute solution of caustic soda boiled in them, followed by a thorough washing in clean water; draining as in the case of the bottles, taking place neck downwards.
A good draining-rack for forcing-flasks is made by stretching two stout copper wires about 1 ½ to 2 inches apart over the sink. IT is well to fill the forcing-flasks within twenty-four hours of taking the sample, and before filling they should be washed out twice with the beer to be sampled. When filled, the neck of the bottle is closed by a small India-rubber stopper which has first had some of the beer poured over it. It is better to leave about 1/8 inch or so between the level of the beer in the neck of the flask, and the side outlet.
When placed on the tray, the side tube should dip not less than ½ inch into mercury conveniently placed in small beakers, each of which will take five or six flasks standing round it. Another method is to have the side tubes dipping into little troughs of wood or porcelain, filled with mercury; this allows of double rows of flasks on the tray, and utilizes a larger proportion of its surface.
The thermometer on the tray may be placed in a flask of water with some mercury at the bottom, so that the temperature indicated is practically that to which the beer in the forcing flask is subjected.
As the object of these experiments is to see how far a beer may be expected to withstand the variations of temperature to which trade casks are subjected, the temperature at which these samples are maintained is in excess of that usually met with, and the growth of Bacteria and forms other than healthy Saccharomyces is thus considered fostered.
The thermometer on the tray showing a constant temperature of 80˚ F., and the samples duly placed, certain observations may be made during the first few days; thus it is useful to note how long the beer takes to drop bright; if a large quantity of gas is given off quickly, or if the beer remains a long time before secondary fermentation commences.
Next, as to the length of time it is desirable to submit beers to this process. For Stock ales—and it is chiefly for this class of beers that the method of examination is of value—we find three weeks the shortest time practically useful, and where possible should advise four weeks.
When the flask is taken off the tray, the appearance of the sample should be noted, whether bright or not, and especially if there be any growth on the surface of the liquid. The liquid when cool should be decanted, and the specific gravity taken with a small saccharometer; the taste amount of acidity, and any peculiarity of flavour are then noted.
The sediment should be shaken up with the few drops of beer remaining in the flask, and a small drop examined in the usual way under the microscope. The chief points to be observed in the microscopic examination of these samples are:
- The condition of the original yeast (S. cerevisiæ).
- The amount of the new yeast.
- The variety of secondary and wild forms present.
- The presence or absence of Bacteria.
- The forms of Bacteria present.
The inter-comparison of such observations will lead anyone very quickly to form an opinion as to the relative keeping quality of the beers examined, and it is chiefly this relative value that is of service to the practical brewer, as it enables him to decide as to the order in which to send out his stock.
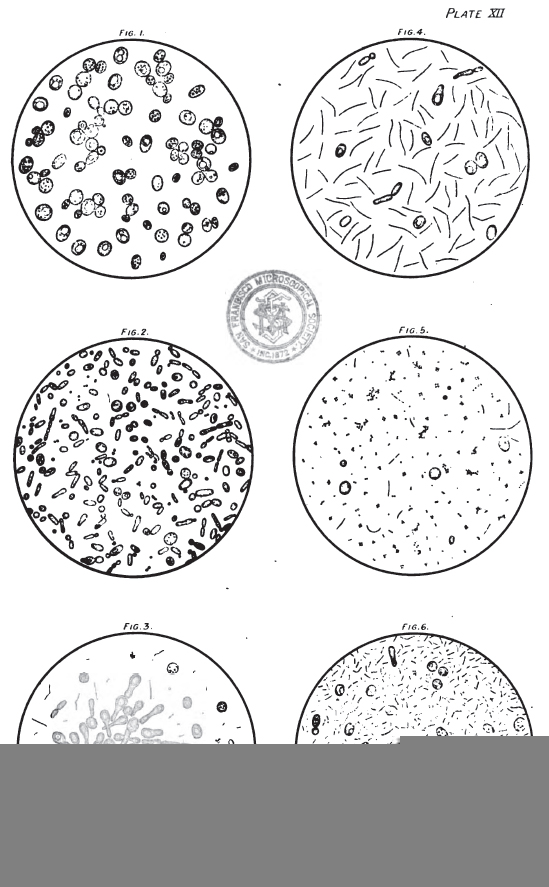
On Plate XII. May be seen examples of forced beer sediments, and it will be noted that the relationship of Saccharomyces to Bacterial forms is variable. From what we have already stated under the separate headings of the Bacteria and Saccharomycetes, it will be concluded that there must necessarily be a great diversity of appearance.
We have found it most convenient to group all forced samples into three classes, I, II, and III, and two subclasses I to II, and II to III. All beers that taste sour when they come off the tray, and that have a high acidity (that is, above the normal but not actually sour), together with a deficiency of new yeast cells, and swarming with short and long Bacterial forms, we mark Class III, and should not consider it safe to keep such beers over six weeks from rack, unless treated with antiseptic. Beers that have no objectionable peculiarity in flavour, and that when examined exhibit a fair amount of new normal yeast forms, yet containing a considerable number of Bacteria, we mark Class I, and should advise them to be put into the trade at an early date, anticipating difficulty with such beers if kept over two months. Sound-tasting beers exhibiting only normal secondary forms of yeast, or no new yeast, and free from Bacteria, or containing only a few Lactic or Bacillus forms in each field, we should mark Class I, and expect to stand well through the summer. Beers that on examination appear too good for Class II and not good enough for Class I we mark I to II, and the same applies to the other sub-class.
The six examples given on Plate XII are fairly typical of this arbitrary but convenient classification:
- Fig I – A normal clean residue of S. Cerevisæ; the large size of some of the cells is probably a result of the forcing tray temperature – Class I.
- Fig 2 – A mixed growth of wild yeasts, chiefly S. Pastorianus and S. Ellipsoideus = Class I to II.
- Fig 3 – An active growth of what is probably Pasteur’s Caseous ferment (S. Coagulatus I.) in a form it is not infrequently met with in forced samples = Class I to II.
- Fig 4 – Few ferment cells, and a vigorous growth of Bacillus subtilis, in the form most frequently seen in these deposits = Class II.
- Fig 5 – A few yeast cells and Bacteria and a considerable growth of Sarcina, probably Pediococcus acidi lactici = Class II.
- Fig 6 – Hardly any yeast growth, and swarming with rod and Bacillus forms, probably B. lactic and B. Subtilis. If not above normal acidity = Class II to III. If markedly acid = Class III.
The gas supply should be of a uniform and steady character, as one of the effects of the gas going out, and the tray consequently falling temperature, is that mercury is driven back into the flasks, especially in the case of flat ales. In so far as the effect of the mercury itself is concerned, we have some reason to believe that it acts as an antiseptic, deterring the production and growth of Bacteria, and possibly of Saccharomyces; but it is a different thing if air enters as well, as a clouding of the beer and film growth on the surface, together with acidity and occasionally ropiness, may ensue, and the experiment be altogether vitiated.
We have also found the tray a suitable apparatus for testing the tendency of malt extracts to become acid, by comparison of the amount of acidity formed in a given number of hours—from 72 to 120—with the normal acidity of the malt extract; a free exposure to air and consequent infection being first permitted. We are of opinion that a useful factor in the determination of the quality of various samples of malt is thus obtained.
Another useful purpose to which the forcing tray may be placed is testing samples of water for purity by the so called Heisch’s test; in so far as the amount of Phosphates in a water is indicative of contamination.
 Pasteur Brewing Louis Pasteur – Science, Health, and Brewing
Pasteur Brewing Louis Pasteur – Science, Health, and Brewing